These unattractive salt deposits mainly consist of water-soluble salts originating from various potential sources.
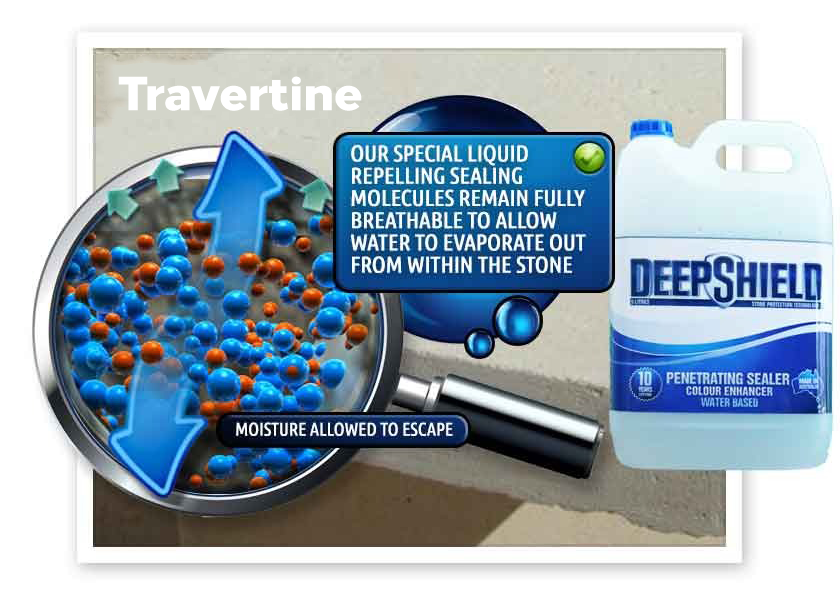
Efflorescence requires the presence of water to dissolve and transport salts. For water to transport salts to the surface in Bunbury, there must be channels through which they can move and migrate. The denser the travertine, the harder it is for water to transport salts to the surface. Conversely, the more porous the material, the easier it is for salts to be transported and deposited.

When salt-bearing water reaches the surface of a travertine structure in Bunbury, it evaporates and leaves behind salt deposits. In low humidity conditions, water may evaporate before reaching the surface, resulting in salt deposits remaining unseen beneath the surface. Conversely, higher humidity slows down water evaporation, providing more opportunities for visible growth of salt deposits.
Two conditions must be present to cause efflorescence in Bunbury:- A source of water-soluble salts.
- Water permeating through the material to transport the salts to the surface. As the water evaporates, it leaves behind the white powder known as efflorescence.
- They may exhibit higher permeability, encouraging water movement.
- Some batches may contain elevated levels of water-soluble salts.